1. Protein Conjugation
Peptide-Directed Photo-Crosslinking for Site-Specific Conjugation of IgG
Bioconjugate Chemistry, 2018, 29, 3240-3244.
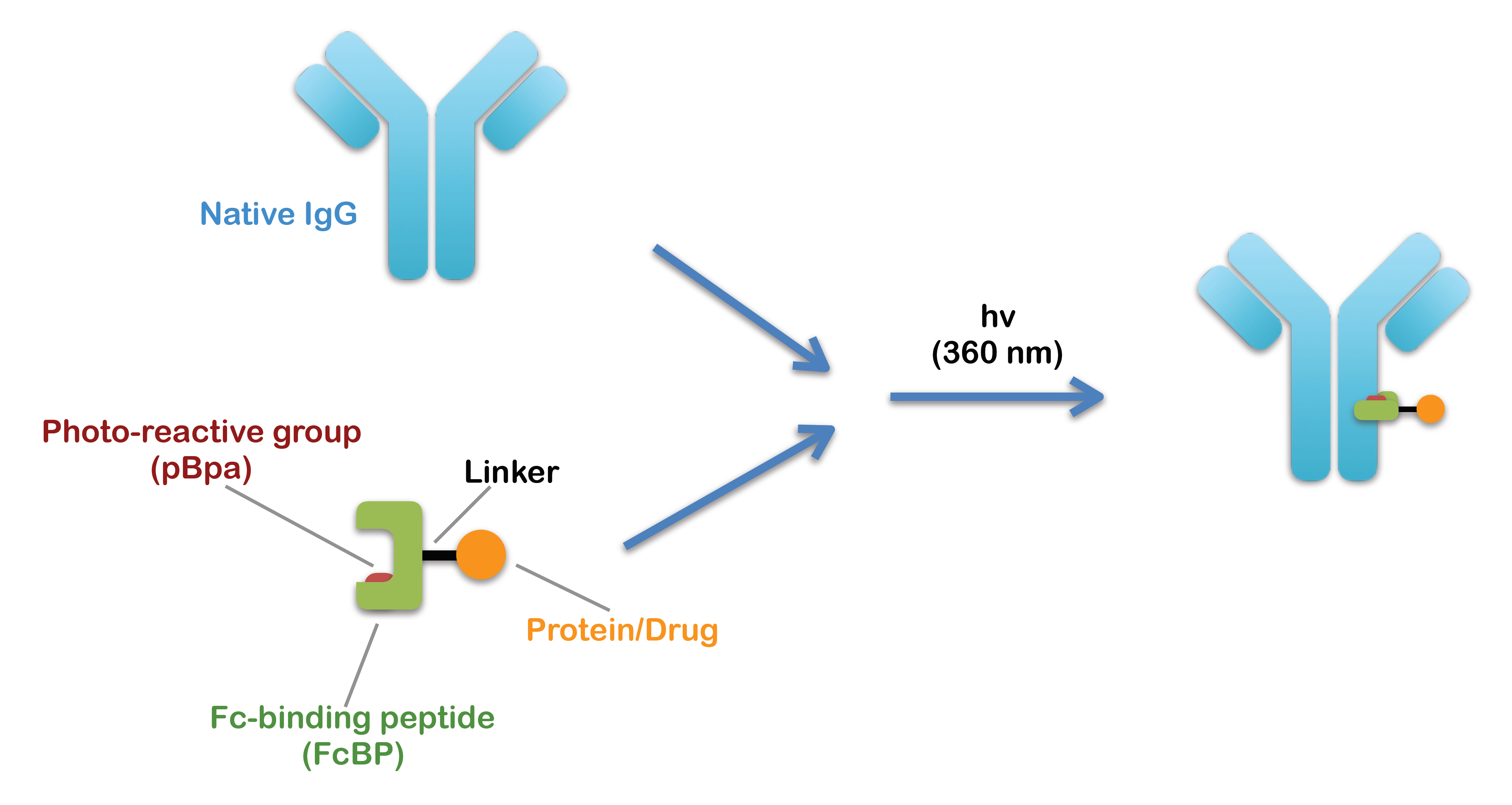
Conjugation of antibody has expanded its applications in therapeutics and diagnostics, and various methods havebeen developed based on chemical or enzymatic reactions. However, the majority of them have focused on synthetic moleculessuch as small molecules, nucleic acids, or synthetic materials, but site-specific conjugation of antibody with protein cargo hasrarely been demonstrated. In this Communication, we report a PEptide-DIrected Photo-cross-linking (PEDIP) reaction for sitespecificconjugation of IgG with protein using an Fc-binding peptide and a photoreactive amino acid analogue, and demonstratethis method by developing an immunotoxin composed of a Her2-targeting IgG (trastuzumab) and an engineered Pseudomonasexotoxin A (PE24). The ADP-ribosylation of eukaryotic elongation factor-2 by the bacterial toxin inhibits the ribosomaltranslation of protein, and the trastuzumab-PE24 conjugate exhibited the cytotoxicity toward Her2-overexpressing cell lines.The PEDIP reaction can also be applied for many other types of cargo with slight modifications of the method.
Incorporation of Unnatural Amino Acids in Response to the AGG Codon
ACS Chemical Biology, 2015, 10, 1648-1653.
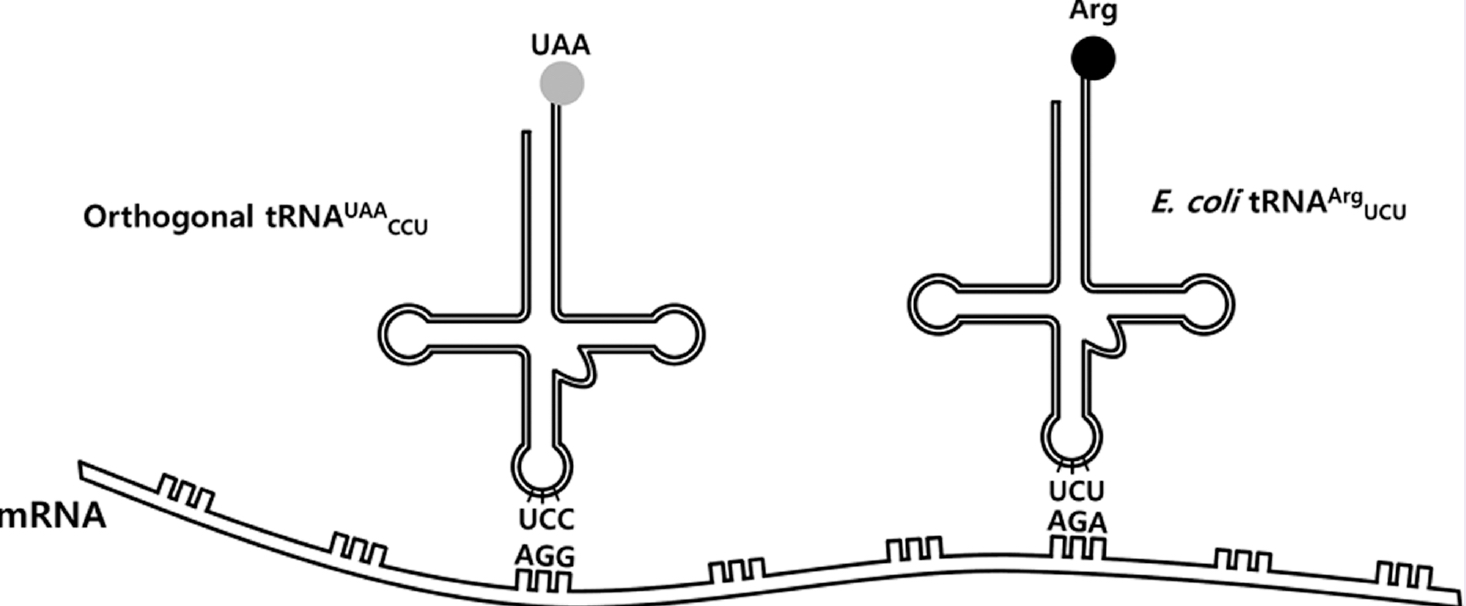
The biological protein synthesis system hasbeen engineered to incorporate unnatural amino acid intoproteins, and this has opened up new routes for engineeringproteins with novel compositions. While such systems havebeen successfully applied in research, there remains a need todevelop new approaches with respect to the wider applicationof unnatural amino acids. In this study, we reported a strategyfor incorporating unnatural amino acids into proteins byreassigning one of the Arg sense codons, the AGG codon.Using this method, several unnatural amino acids werequantitatively incorporated into the AGG site. Furthermore, we applied the method to multiple AGG sites, and even totandem AGG sequences. The method developed and described here could be used for engineering proteins with diverseunnatural amino acids, particularly when employed in combination with other methods.

Synthetic Protein Engineering Laboratory
Department of Molecular Science and Technology, Ajou University
206 Worldcup-ro, Yeongtong-gu, Suwon, 16499 Korea